A) - 602 kJ
B) - 240.8 kJ
C) - 120.4 kJ
D) - 60.2 kJ
E) None of these
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much heat is required to raise the temperature of 25.0 grams of water from 32.0 ° C to 87.0 ° C? Specific Heat (H2O) = 4.184 J/g × ° C
A) 9.20 J
B) 329 J
C) 3.35×103 J
D) 5.75×103 J
E) 1.05×104 J
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the specific heat of an unknown substance if 25.0 grams of the substance absorbs 77.0 calories of heat and the temperature increases by 5.5 ° C?
A) 0.51 cal/g × ° C
B) 0.513 cal/g × ° C
C) 0.56 cal/g × ° C
D) 0.616 cal/g × ° C
E) 0.62 cal/g × ° C
G) C) and E)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Consider the following reaction and its corresponding enthalpy: 2 Mg (s) + O2 (g) →2 MgO (s) D H ° rxn = - 1204 kJ/mol What amount of heat is exchanged when 5.00 grams of magnesium metal reacts at constant pressure?
A) - 124 kJ
B) - 248 kJ
C) - 3.01×103 kJ
D) 7.32×104 kJ
E) 1.44×105 kJ
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heat exchange of a certain exothermic reaction is measured in a solution calorimeter. The sign convention for the heat exchanged in this process would be a ____ value for the heat released in the reaction and a ____ value for the heat absorbed by the calorimeter.
A) negative ( - ) , positive (+)
B) positive (+) , negative ( - )
C) negative ( - ) , negative ( - )
D) positive (+) , positive (+)
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many joules of heat are necessary to raise the temperature of a 1.42 kg block of copper from 25.0 ° C to 88.5 ° C? (Specific heat of copper = 0.385 J/g × ° C)
A) 234000 J
B) 34700 J
C) 234 J
D) 34.7 J
E) 0.0347 J
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following Thermodynamic data:
D H f (Fe2 O3 ; s) = - 822 kJ/mol D H f (FeCl 3 ; s) = - 400 kJ/mol D H f (H₂ O; g) = - 242 kJ/mol D H f (H₂ O; 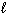 ) = - 286 kJ/mol D H f (HCl; g) = - 92 kJ/mol D H f (HCl; aq) = - 167 kJ/mol What is the Standard Molar Enthalpy of Reaction D H rxn for the following reaction?
Fe2 O3(s) + 6 HCl (g) 2 FeCl 3 (s) + 3 H₂ O (g) D H rxn = ??
) = - 286 kJ/mol D H f (HCl; g) = - 92 kJ/mol D H f (HCl; aq) = - 167 kJ/mol What is the Standard Molar Enthalpy of Reaction D H rxn for the following reaction?
Fe2 O3(s) + 6 HCl (g) 2 FeCl 3 (s) + 3 H₂ O (g) D H rxn = ??
A) - 1256 kJ/mol
B) - 284 kJ/mol
C) - 152 kJ/mol
D) 272 kJ/mol
E) 298 kJ/mol
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a 83.0 g sample of a metal at 100.0 ° C is added tO45.0 g of water at 23.25 ° C, the final temperature of both the water and metal is 27.68 ° C. The specific heat of water is 4.184 J/g × K. What is the specific heat of the metal?
A) 10.0 J/g × K
B) 11.5 J/g × K
C) 0.100 J/g × K
D) 0.139 J/g × K
E) none of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the data below, what is D H ° for the following reaction? CH4 (g) + 3 Cl2 (g) →CHCl3 (g) + 3 HCl (g) D H ° f [CH4 (g) ] = - 75 kJ/mol D H ° f [CHCl3 (g) ] = - 103 kJ/mol D H ° f [HCl (g) ] = - 92 kJ/mol
A) - 304 kJ
B) - 120 kJ
C) +304 kJ
D) +120 kJ
E) none of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions absorbs heat from the surroundings in order to proceed to completion? I. CO (g) + 1/2 O2 (g) →CO2 (g) D H rxn = - 283.0 kJ II. Al2O3 (s) →2 Al (s) + 3/2 O2 (g) (endothermic) III. SO3 (g) + 99.2 kJ→S (s) + 3/2 O2 (g)
A) I only
B) I and III
C) I and II
D) II and III
E) All of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a 50.0 g sample of a metal at 100.0 ° C is added tO60.0 g of water at 25.10 ° C, the final temperature of both the water and metal is 32.81 ° C. The specific heat of water is 4.184 J/g × K. What is the specific heat of the metal?
A) 0.576 J/g × K
B) 1.66 J/g × K
C) 45.0 J/g × K
D) 0.900 J/g × K
E) none of these
G) A) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
The reaction of 8.00 g of SO2 (g) burns in excess oxygen. 9.85 kJ of heat are released. What is D H for the following thermochemical equation? 2 SO2 (g) + O2 (g) →2 SO3 (g)
A) - 1.58×102 kJ
B) - 78.8 kJ
C) - 39.4 kJ
D) +78.8 kJ
E) none of these
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a 2.00 g sample of ammonium nitrate (NH4NO3) dissolves in 48.0 g of water the temperature changes from 26.57 ° C to 23.50 ° C. Assuming the specific heat of the solution is 4.184 J/g × K, what is the enthalpy change for dissolving 2.00 g of ammonium nitrate in water?
A) +616 J
B) +642 J
C) - 642 J
D) - 616 J
E) none of these
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances is expected to have a standard molar enthalpy of formation D H ° f equal to zero?
A) NaCl (s)
B) H2 (g)
C) HCl (aq)
D) N₂( ![]() )
)
E) CO2 (s)
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much heat must be removed from a 10.0 g sample of graphite (specific heat = 0.720 J/g × K) to decrease its temperature from 55.00 ° C tO44.00 ° C?
A) 0.720 J
B) 7.20 J
C) 7.92 J
D) 79.2 J
E) none of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the final temperature upon mixing 75.0 grams of water held at 45.0 ° C witH₂ 5.0 grams of water held at 90.0 ° C?
A) 11.3 ° C
B) 28.1 ° C
C) 56.3 ° C
D) 67.5 ° C
E) 78.8 ° C
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction of 3.44 g of H2 (g) and 20.5 g of C (s) yields C3H6 (g) , and the enthalphy change is - 11.6 kJ. What is D H for the following reaction? 3 H2 (g) + 3 C (s) →C3H6 (g)
A) - 20.4 kJ
B) - 11.6 kJ
C) - 31.7 kJ
D) - 61.2 kJ
E) none of these
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which unit from those listed below would be the best choice as a unit for the heat capacity of a substance?
A) J
B) J/mol
C) J/g
D) J/ ° C
E) J/g × ° C
G) A) and B)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Which of the following defines the heat of formation of Fe2O3 (s) ?
A) 2 Fe (g) + 3 O (g) → Fe2O3 (s)
B) 2 Fe (s) + 3/2 O2 (g) → Fe2O3 (s)
C) 4 Fe (s) + 2 O3 (g) → 2 Fe2O3 (s)
D) 2 Fe (g) + 3 O (g) → Fe2O3 (g)
E) none of these
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the equation below, what is D H for the reaction that converts 6.00 g of H₂ O ( 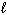 ) into H₂ O₂ (
) into H₂ O₂ ( 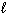 ) and H₂ (g) ?
2 H₂ O (
) and H₂ (g) ?
2 H₂ O ( 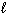 ) H₂ O₂ (
) H₂ O₂ ( 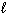 ) + H₂ (g) D H = 384 kJ
) + H₂ (g) D H = 384 kJ
A) 129 kJ
B) 64.0 kJ
C) 256 kJ
D) 384 kJ
E) none of these
G) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 78
Related Exams